Global Presence
Global Presence
We owe our profound gratitude to our stakeholders, to whom we have been able to establish a robust, effective and distinctive network of supply chains across the globe.
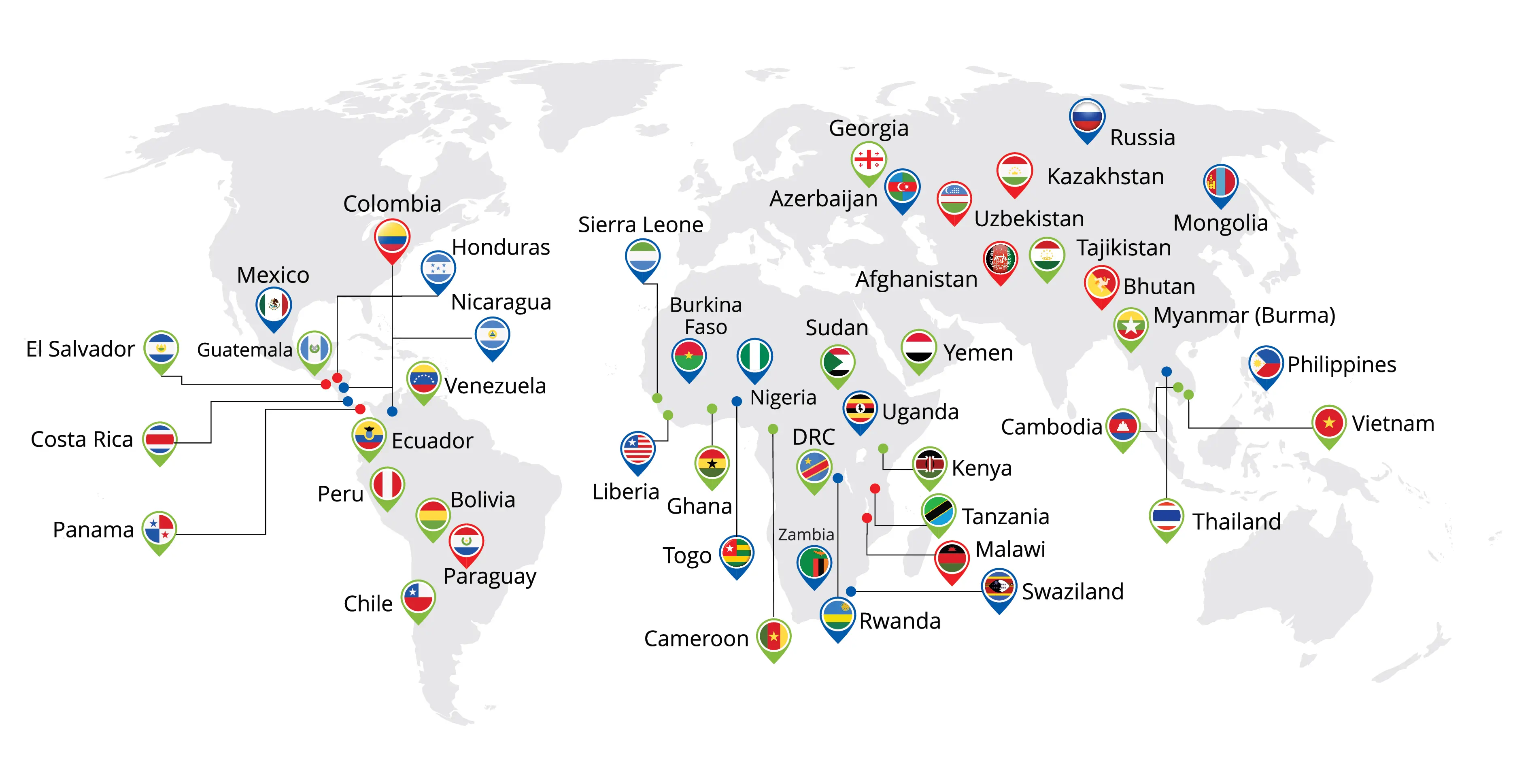
| No | Country Name | Regulatory Authority | ||
|---|---|---|---|---|
| 1 | Afghanistan | GDPA |  |
|
| 2 | Bhutan | DRA |  |
|
| 3 | Bolivia | AGEMED |  |
|
| 4 | Burkina Faso | MOH | ||
| 5 | Cambodia | DDF |  |
|
| 6 | Cameroon | FDA |  |
|
| 7 | Congo | MOH | ||
| 8 | Chile | MOH |  |
|
| 9 | Costa Rica | MOH |  |
|
| 10 | Ecuador | MOH | ||
| 11 | El Salvador | DNM |  |
|
| 12 | Ghana | FDA | ||
| 13 | Guatemala | MOH |  |
|
| 14 | Georgia | LEPL | ||
| 15 | Honduras | ARSA |  |
|
| 16 | Jordan | |||
| 17 | Kazakhstan | CRATIA | ||
| 18 | Kenya | PPB | ||
| 19 | Krygystan | CRATIA | ||
| 20 | Kuwait | MOH | ||
| 21 | Liberia | LMHRA |  |
|
| 22 | Malawi | PMPB |  |
|
| 23 | Mali | DPM | ||
| 24 | Iraq | MOH |  |
|
| 25 | Myanmar | FDA |  |
|
| 26 | Nicaragua | Minsa | ||
| 27 | Nigeria | NAFDAC | ||
| 28 | Panama | MOH |  |
|
| 29 | Paraguay | NDSS | ||
| 30 | Peru | DIGIMED |  |
|
| 31 | Phillipines | PFDA |  |
|
| 32 | Rawanda | FDA | ||
| 33 | Saudi Arabia | SFDA |  |
|
| 34 | Sierra Leone | PBSL |  |
|
| 35 | Sudan | MOH |  |
|
| 36 | Tajikistan | CRATIA | ||
| 37 | Tanzania | TFDA | ||
| 38 | Togo | MOH | ||
| 39 | Uganda | NDA | ||
| 40 | Uruguay | JND | ||
| 41 | Uzbekistan | CRATIA | ||
| 42 | Venezuela | NIH |  |
|
| 43 | Vietnam | DAV |  |
|
| 44 | Yemen | SBD | ||
| Others | ||||
| WHO- GMP |  |
|||
| ISO |  |
|||
| FSSAI |  |
|||
| PHARMAEXCIL PHARMACEUTICALS EXPORT PROMOTION COUNCIL OF INDIA |  |
|||
| HALAL |  |
|||
| HACCP |  |
|||
